• Acellular tissue allograft
• Contains loosely woven collagens
• Terminally Sterilized – SAL 10-6
• 4-Year shelf-life stored at ambient temperature
• Ready-to-Use: does not require re-hydration or rinsing of preservation media
• Requires no specific orientation
• Highly conformable
• To serve as a cover
• To protect from the surrounding environment
• To retain fluid
• Open Procenta box and remove the product pouch
• Using aseptic technique, peel open the outer pouch containing the sterile vial
• Open vial by the tear-off tab and remove the rubber stopper
• Remove product from the vial and apply to the treatment site using instrument such as a forceps
• Because Procenta is completely conformable, it requires no specific orientation on the treatment site
• Apply Procenta evenly over the surface of the wound
• Cover wound site with a non-adherent, impregnated dressing
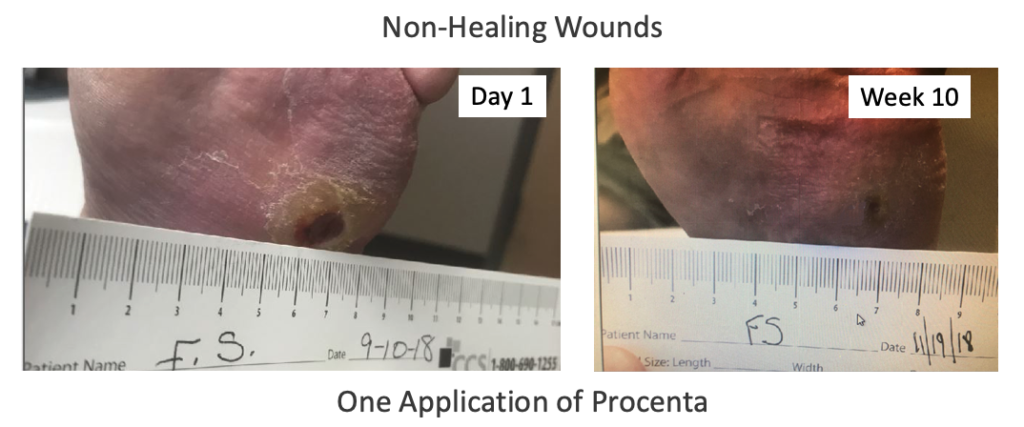
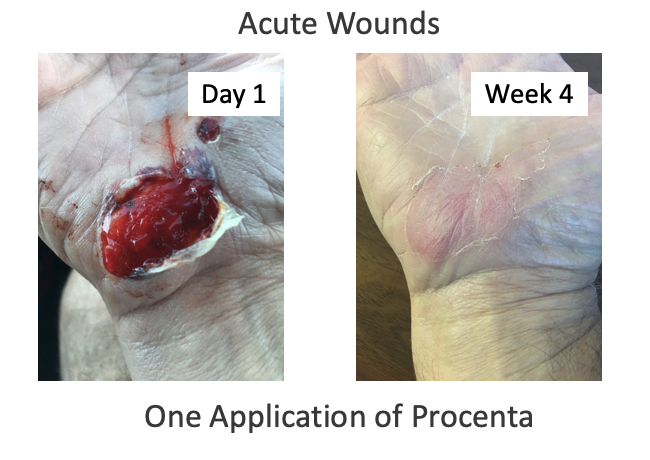
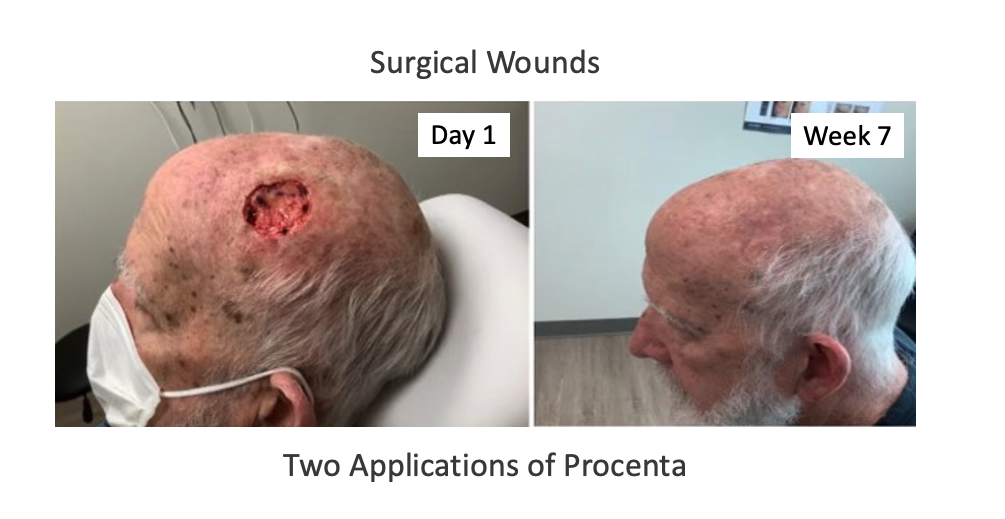
Introducing Nativus®, an innovative processing system created to retain the native structural components of human placental tissues while strictly adhering to FDA regulations, particularly CFR Title 21, Part 1271, Section 361. Nativus® protects the native extracellular matrix components of tissue without freezing, heating, dehydration, chemical treatment, or micronization/morselizing steps.
Both birth mother and donated tissue are thoroughly screened and tested to ensure that the product is safe for transplantation. To further minimize risk, it is terminally sterilized.
When the tissue is processed, cellular components (including blood cells) are removed to prevent the chance of tissue rejection. Anti-rejection drugs are not required for the transplant recipient.
Yes, Procenta® is FDA approved.

